Monocyte Activation Test Mat

It was found that all hsa batches were contaminated with 1 3 beta glucans which interfere with the conventional lal.
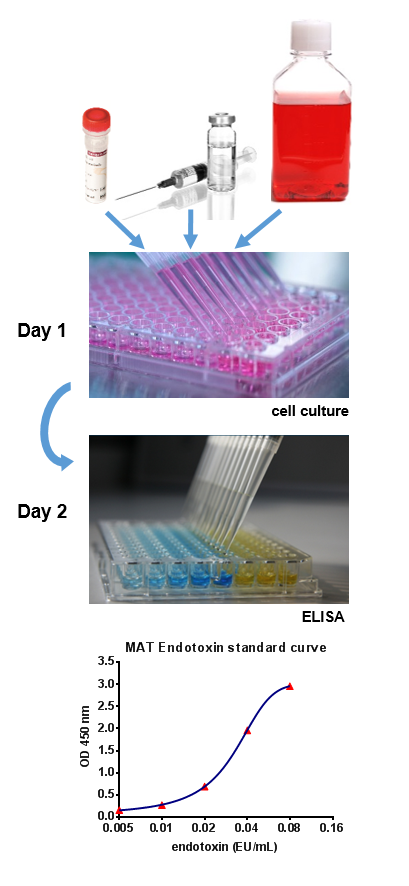
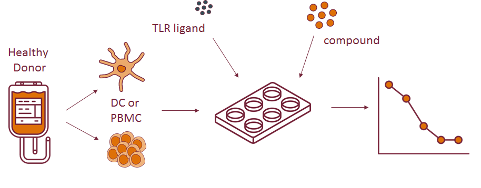
Monocyte activation test mat. Supported by many regulatory bodies the robust mat assay produces sensitive results based on the human immune reaction. Application note detection of pyrogens in hormone based drugs. It mimics human reaction to pyrogens by using cryopreserved human blood making sure it s highly reliable and robust. The mat is suitable after product specific validation as a replacement for the rpt.
In the current study 16 hsa batches were assayed for pyrogens in parallel with the rabbit pyrogen test conventional and endotoxin specific lal assay and monocyte activation test mat. Ctl mat llc was founded by drs. Drs solati serves as ceo. At nibsc a monocyte activation test mat was adapted and validated as an alternative.
The monocyte activation test mat can detect both endotoxin and non endotoxin pyrogens in one test. Dr lehmann is the cso of ctl mat and is also the founder and ceo of cellular technology limited. She has over 14 years of monocyte activation test experience with both the scientific and regulatory aspects of the technology. Shabnam solati and dr.
Two mat systems for pyrogen testing including endotoxin and non endotoxin pyrogen nep detection. We offer a ready to use kit for the monocyte activation test mat. This required setting of a specification in house and deciding on a decisional procedure using multiple donors allowing batches equally pyrogenic or less than those batches shown to be safe in a clinical trial to be certified as safe. The mat is a rabbit free alternative to the rabbit pyrogen test rpt and can be used to test parenteral drugs for endotoxin and non endotoxin contaminations.
Wickham laboratories is pleased to announce the introduction of a new in vitro test method the monocyte activation test mat for the assessment of pyrogenicity of pharmaceutical products and medical devices. Application note comparison of reference standard endotoxins rse application note detection of pyrogens in fbs with pyromat. Application note detection of nep by mat using the pyromat system. Reflecting our strong commitment to the 3rs charles river continuously seeks new methods and technologies to provide clients with viable in vitro alternatives to in vivo tests.
White paper mat statistical analysis. White paper monocyte activation test.