Mat Test For Pyrogens

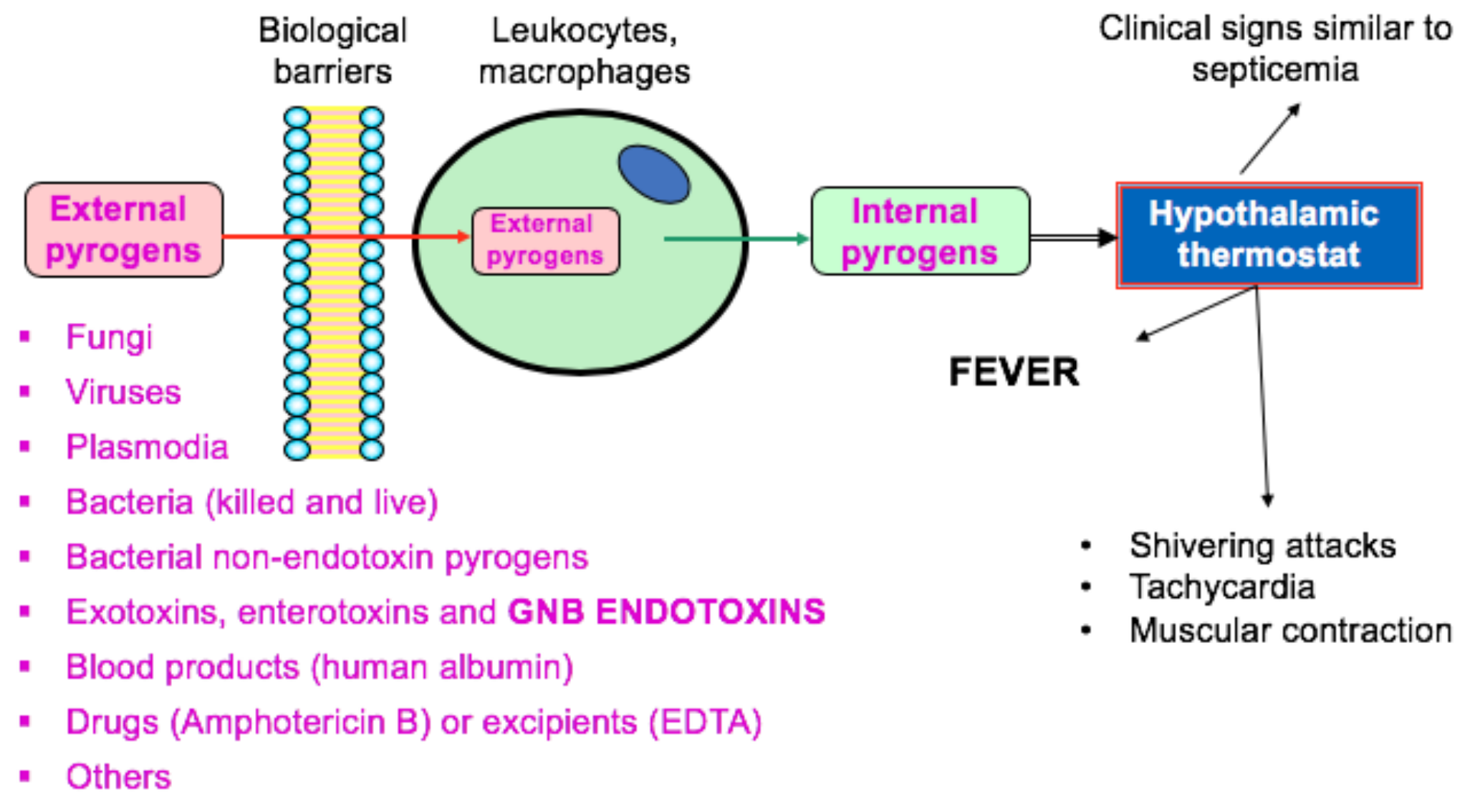
Material mediated pyrogens mmps while less common may also be present.
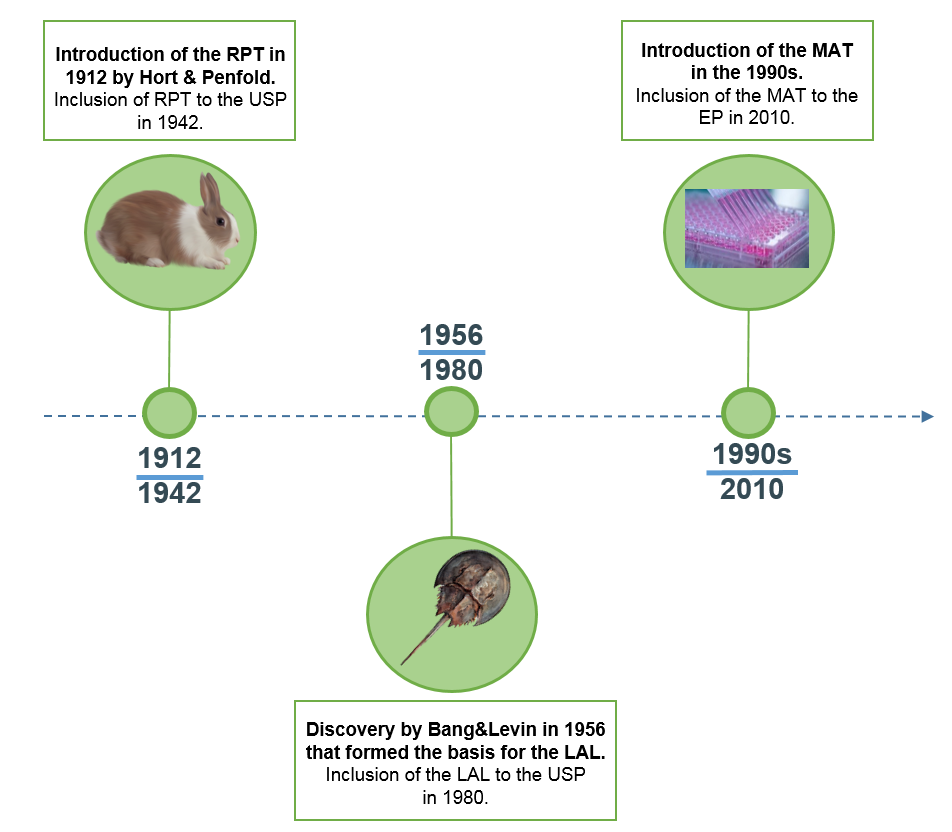
Mat test for pyrogens. Monocytes are exposed in tissue culture to the test substance. If the risk assessment indicates that non endotoxin pyrogens may be present it may be more. Based on human immune cells the mat detects all pyrogens. Since 2010 the european pharmacopoeia ep considers the mat as a replacement for the rabbit pyrogen test ep chapter 2 6 30.
It mimics human reaction to pyrogens by using cryopreserved human blood making sure it s highly reliable and robust. Mimicks the humane immune response. Can analyse pyrogenic activity in more complex pharmaceuticals like. For devices and drug materials firms should assess the risk of the presence of non endotoxin pyrogens.
Most pyrogens are biological substances derived from bacteria fungi and viruses. Avantages of the monocyte activation test. Supported by many regulatory bodies the robust mat assay produces sensitive results based on the human immune reaction. In vitro pyrogen test methods.
The monocyte activation test mat method description. Detects all pyrogens relevant to humans. The mat is a rabbit free alternative to the rabbit pyrogen test rpt and can be used to test parenteral drugs for endotoxin and non endotoxin contaminations. Two mat systems for pyrogen testing including endotoxin and non endotoxin pyrogen nep detection.
The benefits of mat as a pyrogen test. Pyrogens are substances that can produce fever when present as contaminants in a drug or medical device. More flexible than the most frequently applied methods rpt and bet or lal. If the test substance contains pyrogens these will elicit il 6 production in the monocytes which can be detected by an il 6 elisa in the culture supernatant.
Free webinars for pyrogen testing. Detection of the full range of pyrogens including endotoxin and non endotoxin pyrogens in one test. It was found that all hsa batches were contaminated with 1 3 beta glucans which interfere with the conventional lal. The monocyte activation test an in vitro pyrogen test is suitable to replace the rpt lal and rfc.
Extended range of products can be tested. In the current study 16 hsa batches were assayed for pyrogens in parallel with the rabbit pyrogen test conventional and endotoxin specific lal assay and monocyte activation test mat. In vitro no animals are harmed. Pyrogens fever endotoxin non endotoxin pyrogen nep monocyte pharmacopeia bacterial endotoxin test bet monocyte activation test mat synergisms.