Mat Pyrogen Test

We offer a ready to use kit for the monocyte activation test mat.
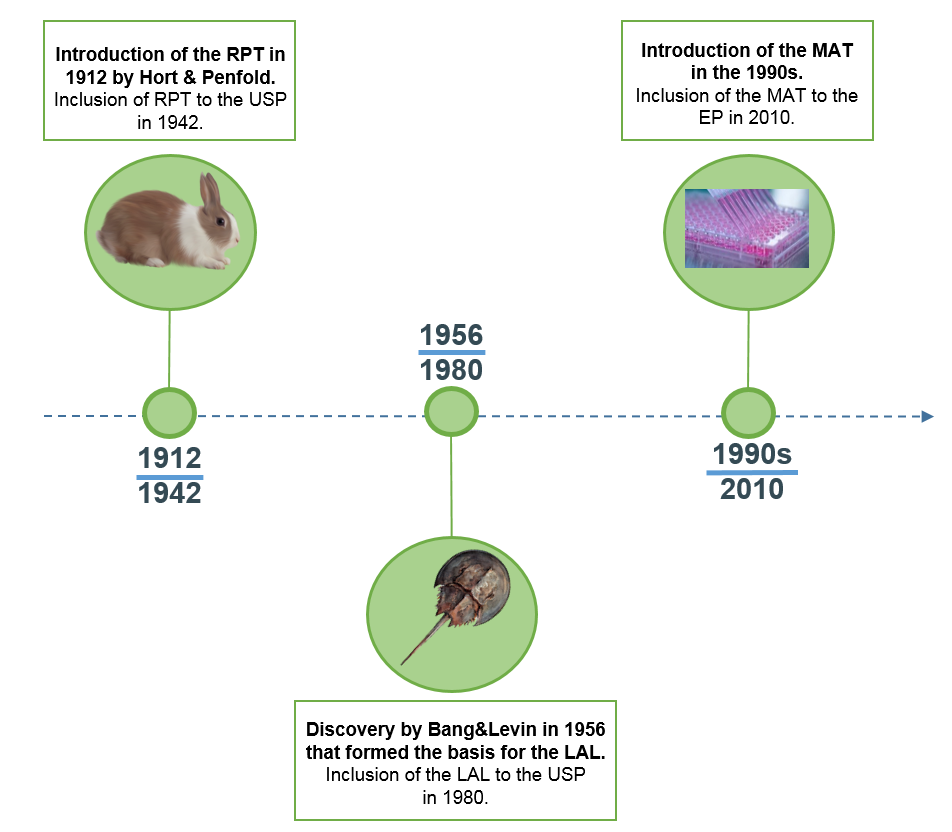
Mat pyrogen test. The mat works by predicting the human response to pyrogens on the basis of human fever and may be used as an alternative to the rabbit pyrogen test. As of 2010 the monocyte activation test has been pointed to as the compendial method of pyrogen detection in the european. The rabbit pyrogen test rpt people throughout the world should be able to count on parenteral drugs cosmetics and biopharmaceuticals to be safe. This assay can be used to detect gram positive and gram negative organisms and parasitic viral and other biological pyrogens e g yeast.
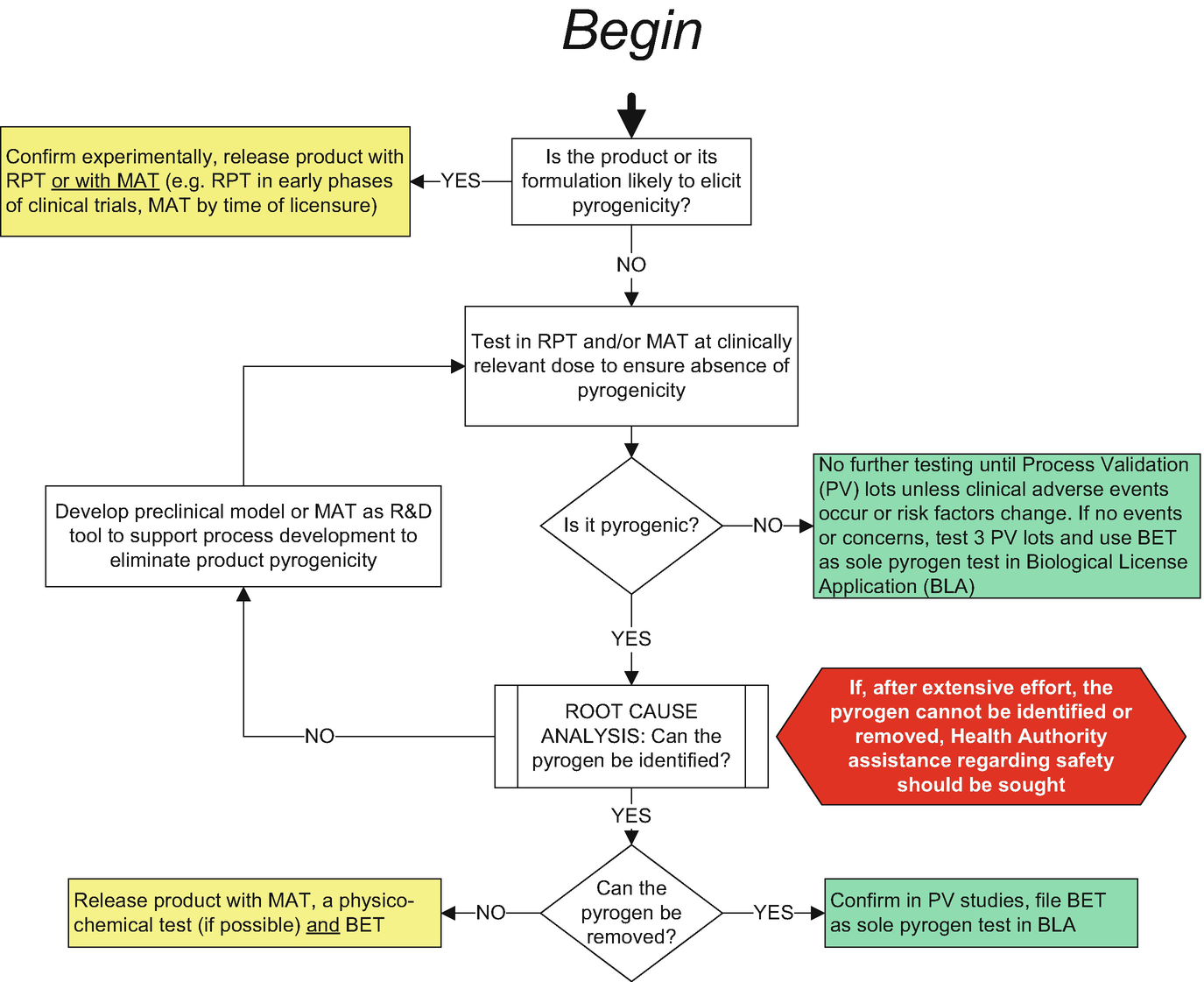
In 2008 iccvam evaluated the status of five proposed in vitro test methods all types of mat to replace the rabbit pyrogen test and concluded that none of the proposed test methods could completely replace the rabbit pyrogen test to detect gram negative endotoxin a common pyrogenic substance. Supported by many regulatory bodies the robust mat assay produces sensitive results based on the human immune reaction. However the in vitro tests can be considered to. In the current study 16 hsa batches were assayed for pyrogens in parallel with the rabbit pyrogen test conventional and endotoxin specific lal assay and monocyte activation test mat.
Pharmacopoeia chapter 2 6 30 and since the 2016 revision recommendations have been given to replace tests on rabbits with the mat wherever possible and after product specific validation ep 2 6 8 rev. The monocyte activation test mat vs. The mat is a rabbit free alternative to the rabbit pyrogen test rpt and can be used to test parenteral drugs for endotoxin and non endotoxin contaminations. Everything you need to start using mat as a pyrogen test.
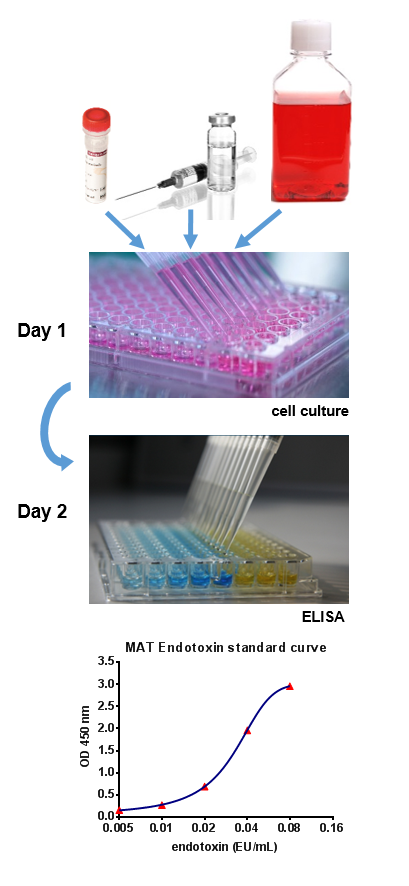
Rabbit free testing of a wider range of pyrogens. A highly sensitive in vitro human cell based assay for pyrogen detection. It was found that all hsa batches were contaminated with 1 3 beta glucans which interfere with the conventional lal. The assay is based on the human immune response by measuring cytokine production of human peripheral blood mononuclear cells pbmc.
With the highest reactivity sensitivity reproducibility and batch sizes of 2 000 mat biotech s monocyte activation test mat kit provides the best performing pyrogen test available on the market. Monocyte activation test mat. Microbial contamination however can happen even under the most stringent manufacturing standards. In recent years an alternative in vitro pyrogen test the monocyte activation test mat has been developed to detect and quantify endotoxin and nep contaminations.
The same holds true for medical devices.